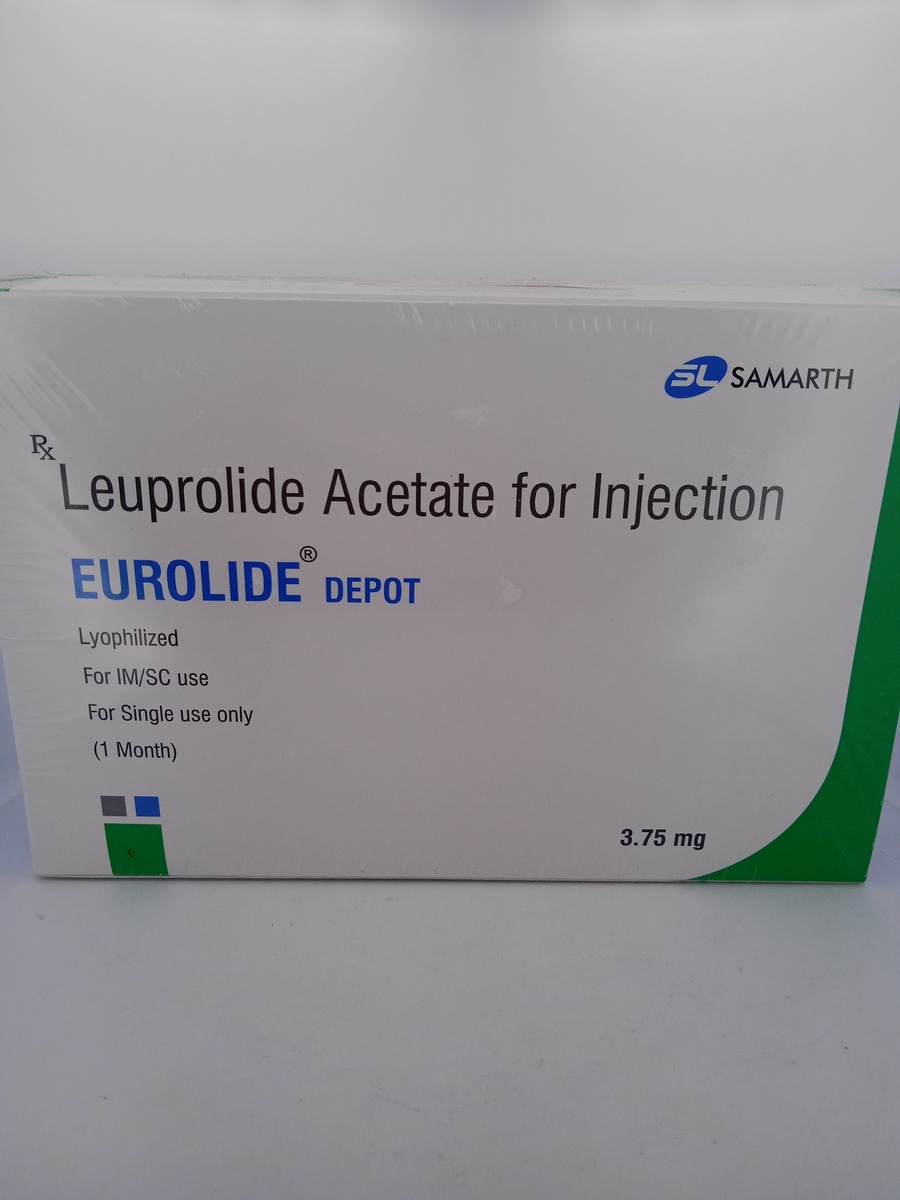
EUROLIDE DEPOT 3.75 MG contains leuprolide acetate, a synthetic gonadotropin-releasing hormone (GnRH) analog. This medication is administered via intramuscular injection, providing a controlled release over time. By suppressing the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland, it effectively reduces the production of testosterone in men and estrogen in women. This hormonal modulation is beneficial in treating prostate cancer by slowing tumor growth and alleviating symptoms associated with endometriosis and uterine fibroids. Additionally, it helps manage early-onset puberty in children by regulating hormone levels.
Usage Instructions:
Administration: EUROLIDE DEPOT 3.75 MG is administered as an intramuscular injection by a healthcare professional.
Frequency: Typically given once a month, or as directed by your physician.
Follow-Up: Regular follow-ups and hormone level monitoring are recommended to assess the treatment’s effectiveness and make necessary dosage adjustments.
Medicinal Advantages:
Effective for Prostate Cancer: Slows the progression of hormone-sensitive tumors.
Alleviates Endometriosis Symptoms: Reduces pain and other symptoms associated with endometriosis by lowering estrogen levels.
Controls Uterine Fibroids: Helps shrink fibroids and manage symptoms like heavy bleeding and pelvic pain.
Regulates Premature Puberty: Normalizes hormonal levels in children, helping manage early sexual development.
Mechanism of Action:
EUROLIDE DEPOT 3.75 MG acts by mimicking GnRH, initially stimulating the release of LH and FSH. However, continuous administration leads to downregulation of GnRH receptors in the pituitary gland, resulting in decreased secretion of these hormones. This suppression ultimately leads to reduced levels of testosterone in men and estrogen in women, inhibiting the growth of hormone-dependent tissues.
Off-Label Uses:
May be used for treating conditions like advanced breast cancer or precocious puberty not specifically mentioned in the primary indications.
Expert Advice:
Regular Monitoring: Regular blood tests are essential to monitor hormone levels and assess treatment efficacy.
Bone Health: Long-term use may affect bone density; discuss monitoring and potential supplementation with your doctor.
Inform Your Doctor: Always inform your healthcare provider about any other medications you are taking or health conditions you have.
Precautions:
Allergic Reactions: Watch for any signs of hypersensitivity, such as rash, itching, or swelling.
History of Hormone-Sensitive Conditions: Patients with a history of hormone-sensitive tumors should consult their doctor before starting treatment.
Pregnancy and Breastfeeding: EUROLIDE DEPOT should not be used during pregnancy or breastfeeding; consult your doctor for alternatives.
Storage Conditions:
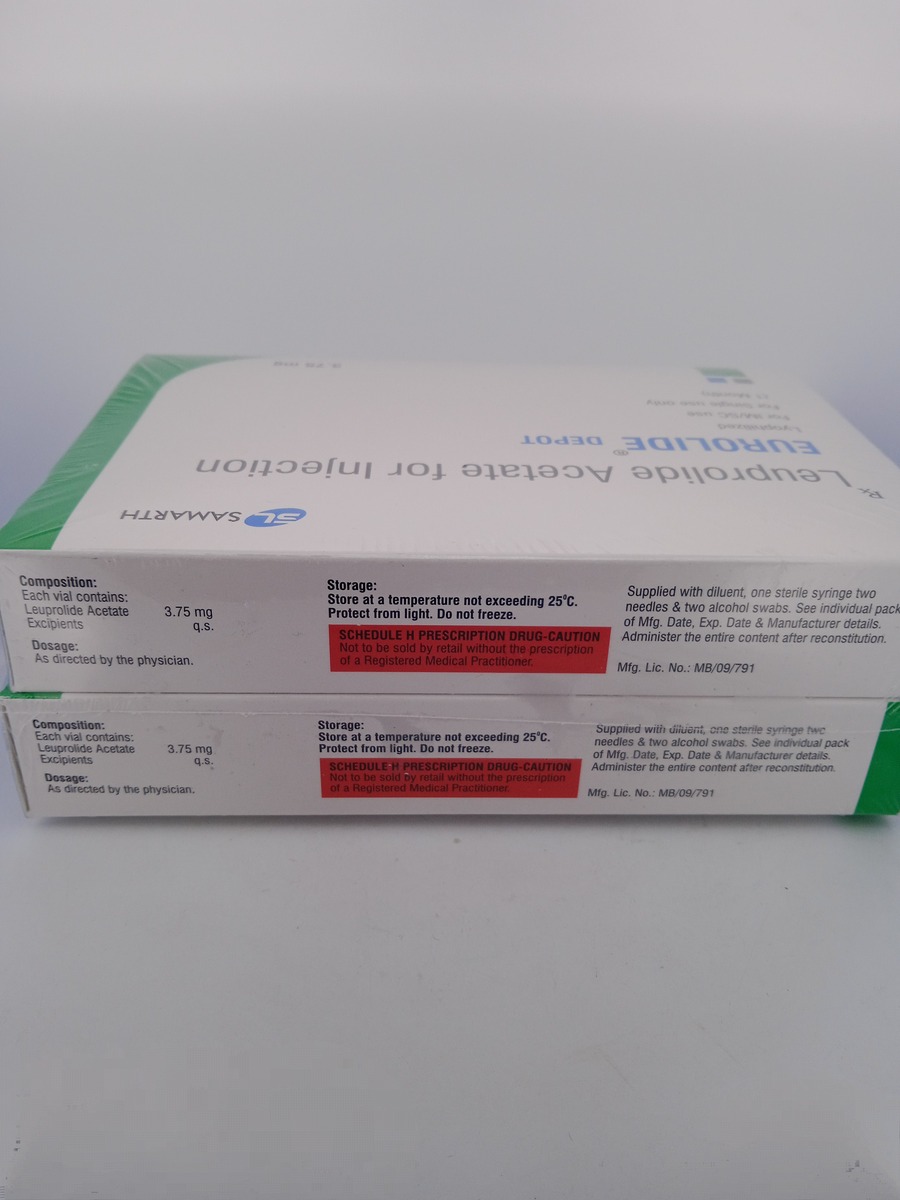
Store EUROLIDE DEPOT 3.75 MG in a refrigerator at 2°C to 8°C (36°F to 46°F).
Do not freeze, and keep the medication protected from light.
Ensure it is out of reach of children and properly dispose of any expired or unused product.
FAQs:
Q1: What is EUROLIDE DEPOT 3.75 MG used for?
A1: It is used to treat prostate cancer, endometriosis, uterine fibroids, and premature puberty by regulating hormone levels.
Q2: How often is EUROLIDE DEPOT administered?
A2: It is usually given once a month as an intramuscular injection by a healthcare provider.
Q3: Can EUROLIDE DEPOT affect fertility?
A3: Yes, it can affect fertility; discuss any concerns with your healthcare provider before starting treatment.
Q4: Are there any side effects?
A4: Common side effects include hot flashes, fatigue, injection site reactions, and changes in mood. Consult your doctor for a complete list.
Q5: Is it safe to use EUROLIDE during pregnancy?
A5: No, it is contraindicated during pregnancy and should only be used under a doctor’s supervision.





Reviews
There are no reviews yet.